Share Your Passion for Research with a New GenerationContinue reading
TGen and Mayo Clinic help launch national clinical trials to combat advanced skin cancer
Arizonans will receive benefit from recently FDA-approved precision medicine clinical trial to fight deadly melanoma Continue reading
Beating Advanced Cancers: New Epigenomic Block for Advanced Cancer Progression
An international research team led by Mayo Clinic oncologists has found a new way to identify and possibly stop the progression of many late-stage cancers, including bladder, blood, bone, brain, lung and kidney.Continue reading
ASU researchers search for telltale signs of ovarian cancer
This year, ovarian cancer will claim over 125,000 lives worldwide. The deadly disease remains the fifth leading cause of cancer-related mortality in U.S. women, killing about 15,000 per year.
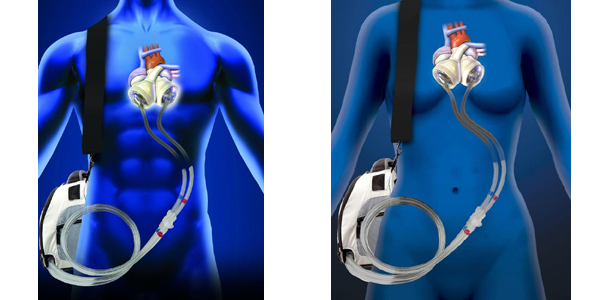
FDA Approves the SynCardia Total Artificial Heart for Destination Therapy Study
19 patients who are not eligible for a donor heart transplant will participate in the pivotal clinical study to evaluate the SynCardia Total Artificial Heart for permanent use.Continue reading
Capstone Therapeutics Announces Phase 1b/2a Study Results for AEM-28 Showing Safety and Biomarker Efficacy Signals
Capstone Therapeutics (OTCQB:CAPS) and its joint venture affiliate, LipimetiX Development, LLC (“JV”), announced today the completion of and results for its investigational AEM-28 (Apo E mimetic peptide) Phase 1b/2a human clinical trial in cholesterol and lipid reduction.Continue reading
Barrow Neurosurgeons Implant the World’s First Scaffold into a Patient’s Spinal Cord
Surgery Performed on 25-Year-Old Valley Man after Dirt Bike Accident
Neurosurgeons at Barrow Neurological Institute have implanted the world’s first scaffolding device into the spinal cord of a patient.
Continue reading
Coming out of the Shadows: EMA approves first treatment for EPP
Perseverance and adaptability are requisite qualities in drug development.
Calimmune (Tucson, AZ) Approved to Treat Second Group in HIV Stem Cell Gene Modification Study
June 25, 2014
San Diego, CA – Calimmune today announced encouraging safety data from its innovative gene-based stem cell therapy, Cal-1-being developed to help cure individuals infected with HIV. The company can now begin treating the second group of patients in the trial, which is being funded in part by a grant from the California Institute for Regenerative Medicine (CIRM).Continue reading
Insys Therapeutics Receives FDA Orphan Drug Designation for Its Pharmaceutical Cannabidiol as a Potential Treatment for Rare Form of Epilepsy, Lennox-Gastaut Syndrome
Insys Therapeutics, Inc. (INSY), a specialty pharmaceutical company that is developing and commercializing innovative drugs and novel drug delivery systems, today announced that the U.S. Food and Drug Administration (FDA) has granted orphan drug designation to its pharmaceutical cannabidiol (CBD) for the treatment of Lennox-Gastaut Syndrome, a rare pediatric-onset epilepsy.